-
Format
Broadcast
- Language English
- Views 14
-
Date
10 Feb 2025
- Price 80.00 €
RUMED Online Training Program:
The complexity of instrument reprocessing and related processes is increasing, requiring a deep understanding of correlations within various workflows. This expertise is crucial for making informed decisions and upholding the high quality standards in our daily work at RUMED. Achieving this requires a strong foundation of technical and procedural knowledge. To meet these demands, the RUMED Academy offers a comprehensive online training program consisting of two levels: 'Reprocessing Technician RUMED (Level I)' and 'Senior Processing Technician RUMED (Level II)'.
The modules are conducted in English, and delivered by experienced international experts with extensive knowledge and practical experience in the field of application in the operating room (OR) and instrument reprocessing. The content of the modules is based on the comprehensive DGSV and HSPA courses, ensuring a well-rounded and relevant training experience.
Reprocessing Technician RUMED (Level II):
The Level II program consists of 9 individual modules (see below “Module Overview”). The target group is RUMED managers, RUMED technicians and other employees in RUMED with proficient English/Spanish language skills and practical experience in a reprocessing structure for medical devices.
Module 9.1: Material and Instrument Sciences II:
The field of Material and Instrument Sciences is crucial in the development and optimization of surgical instruments, where the choice of materials directly impacts the performance, durability, and biocompatibility of these tools. Surgical instruments must withstand repetitive use, sterilization, and exposure to various biological environments without degrading in functionality. Key materials include stainless steel, titanium, and advanced polymers, chosen for their strength, corrosion resistance, and ability to maintain sharpness and precision. Furthermore, the reprocessing of surgical instruments—cleaning, sterilizing, and maintaining—plays a vital role in ensuring their long-term usability and safety. Effective reprocessing methods must be compatible with the materials used in the instruments to prevent damage and ensure complete sterilization. Innovations in material science continue to drive improvements in both instrument performance and the efficacy of reprocessing techniques, ultimately enhancing patient outcomes and reducing the risk of surgical complications.
Certification:
Upon successful completion of this module, a certificate with 3.0 CE point by the HSPA is issued as proof of achievement. Each module concludes with a knowledge quiz. Once you have completed all modules and provided the corresponding certificates to the RUMED Academy (contact@rumedacademy.com), you will be awarded the Reprocessing Technician RUMED (Level II) certificate.
Participation Cost:
€ 80.00 per Module
Module Overview (Level II):
6.1 Structure and Design of RUMED and its Processes II
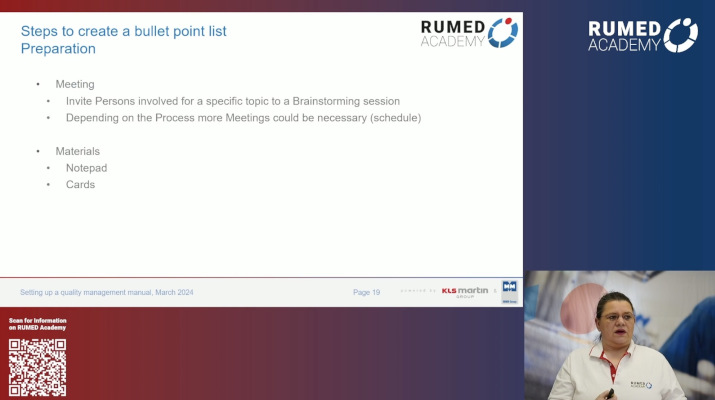
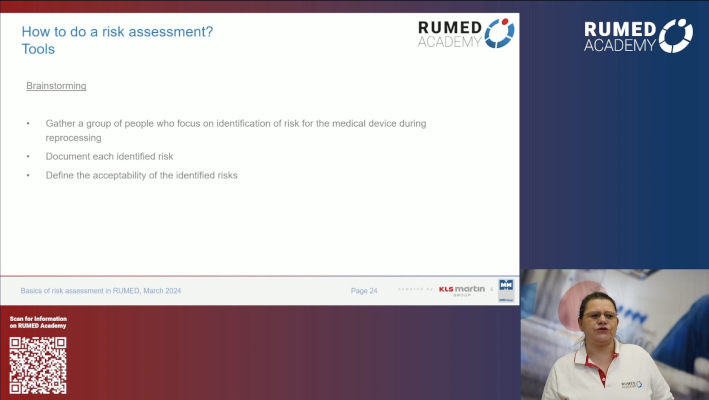
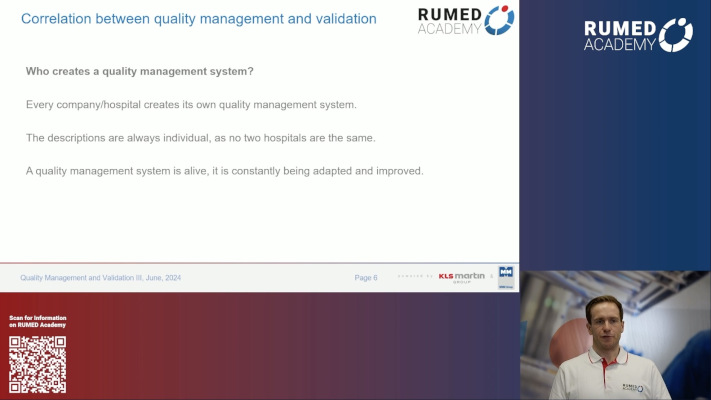
7.1 Quality Management and Validation II
7.2 Quality Management and Validation III
7.3 Quality Management and Validation IV
7.4 Quality Management and Validation V
8.2 RUMED Management III
8.3 RUMED Management IV
9.1 Material and Instrument Sciences II
11.1 Processing of Medical Devices in the Supply Chain II
Module Overview (Level I):
1.0 Standards, Laws and Regulation
2.0 Hygiene and Microbiology
3.0 Cleaning and Disinfection I
3.1 Cleaning and Disinfection II
4.0 Sterilization
5.0 Proper Machinery Working
6.0 Structure and Design of RUMED and its Processes
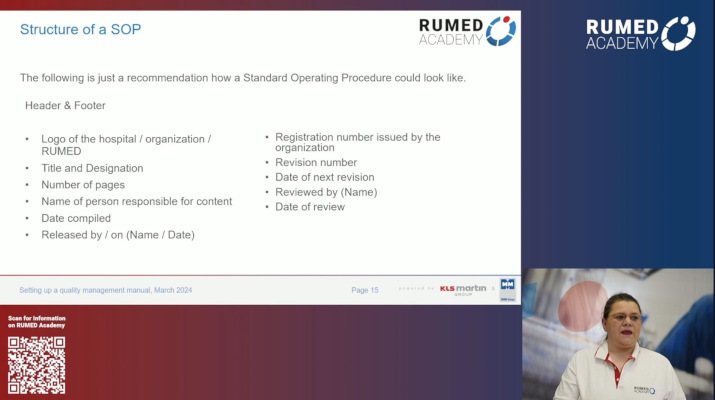
7.0 Quality Management and Validation I
8.0 RUMED Management I
8.1 RUMED Management II
9.0 Material and Instrument Sciences I
10.0 Packaging and Sterile Barrier Systems I
10.1 Packaging and Sterile Barrier Systems II
11.0 Processing of Medical Devices in the Supply Chain I