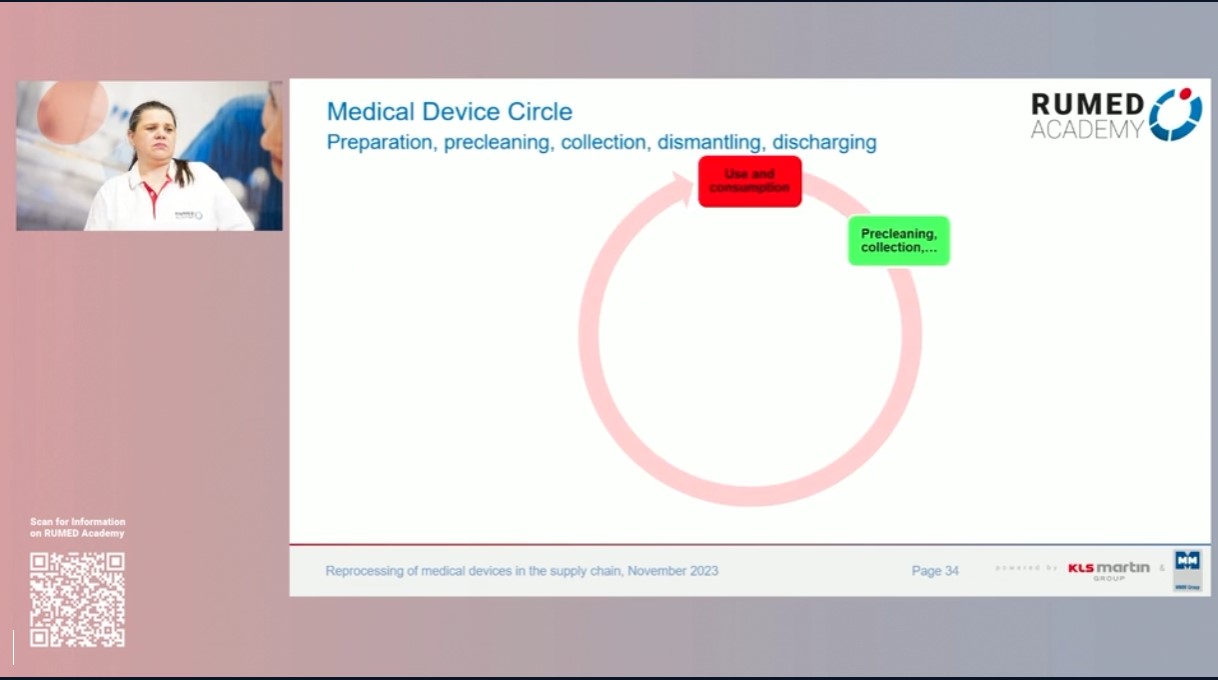
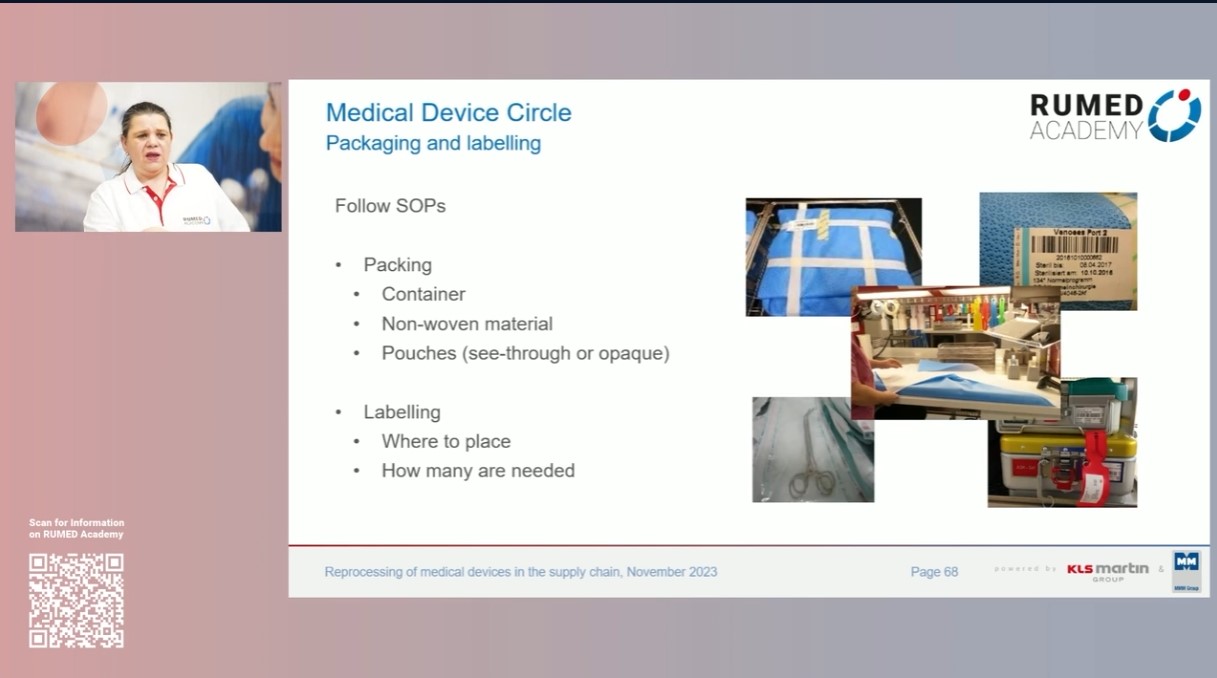
-
Format
Broadcast
- Language English
- Views 133
-
Date
29 Nov 2023
- Price 80.00 €
RUMED Online Training Program:
The complexity of instrument reprocessing and related processes is increasing, requiring a deep understanding of correlations within various workflows. This expertise is crucial for making informed decisions and upholding the high quality standards in our daily work at RUMED. Achieving this requires a strong foundation of technical and procedural knowledge. To meet these demands, the RUMED Academy offers a comprehensive online training program consisting of two levels: 'Reprocessing Technician RUMED (Level 1)' and 'Senior Processing Technician RUMED (Level 2)'.
The modules are conducted in English, available also in Spanish in Latin America, and delivered by experienced international experts with extensive knowledge and practical experience in the field of application in the operating room (OR) and instrument reprocessing. The content of the modules is based on the comprehensive DGSV and HSPA courses, ensuring a well-rounded and relevant training experience.
Reprocessing Technician RUMED (Level I):
The Level I program consists of 14 individual modules (see below “Module Overview”). The target group is RUMED managers, RUMED technicians and other employees in RUMED with proficient English/Spanish language skills and practical experience in a reprocessing structure for medical devices.
Module 7.0: Quality Management and Validation I:
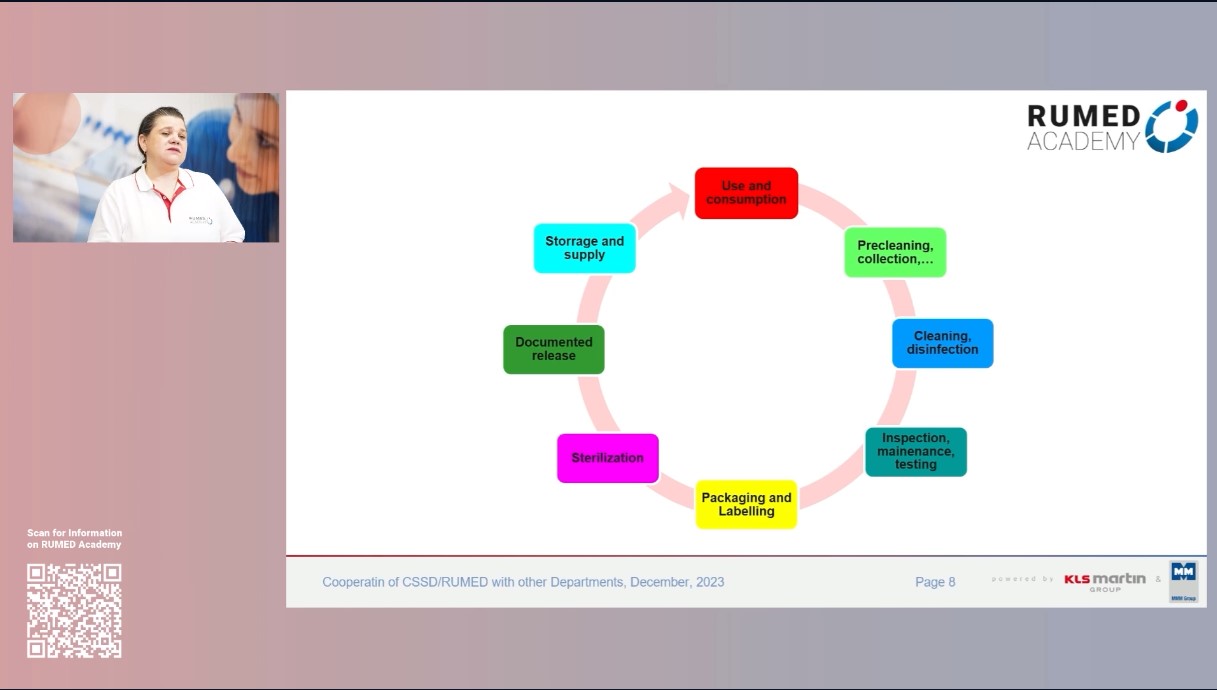
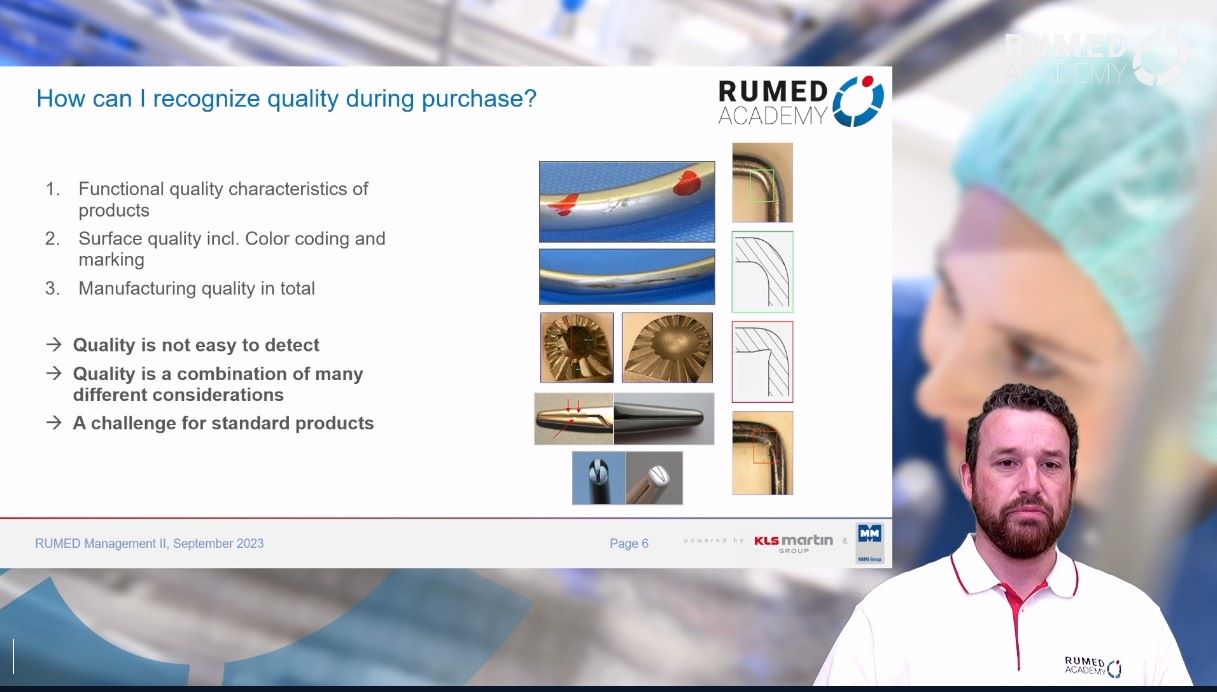
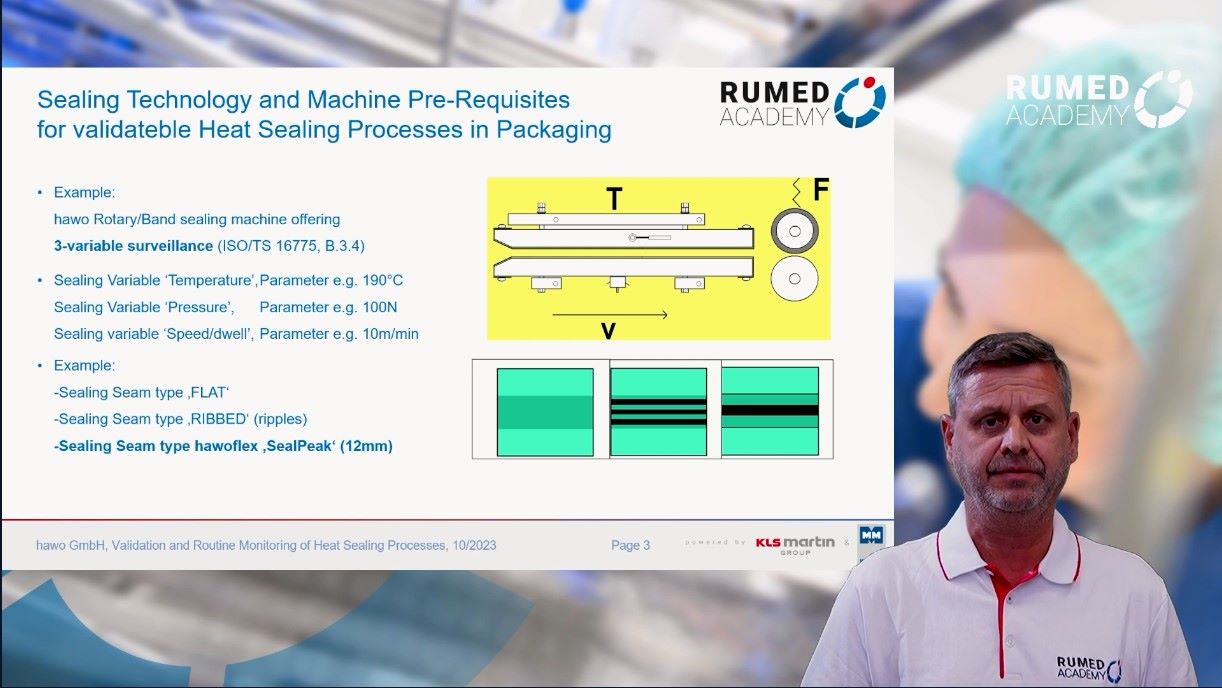
Quality management plays a crucial role in ensuring the safe and efficient functioning of a CSSD. Effective quality management in CSSD includes various aspects e.g., training and competence of employees, compliance with guidelines and standardized procedures, strict monitoring of sterilization and disinfection processes, regular maintenance of equipment, and comprehensive documentation. By implementing quality control measures, the risks of infection can be minimized, errors can be reduced, and patient and customer satisfaction. In addition, quality management in the CSSD contributes to operational efficiency by optimizing resource utilization and streamlining workflows. Regular monitoring and evaluation, as well as continuous quality improvement initiatives, help to identify areas for improvement and drive sustainable improvement in CSSD operations. In conclusion, quality management is critical to ensuring patient safety and maximizing operational efficiency. By establishing quality management and continuous improvement, healthcare organizations can strengthen their CSSD practices, ultimately benefiting both patients and healthcare providers.
Certification:
Upon successful completion of this module, a certificate with 2.5 CE points by the HSPA is issued as proof of achievement. Each module concludes with a knowledge quiz. Once you have completed all modules and provided the corresponding certificates to the RUMED Academy (contact@rumedacademy.com), you will be awarded the Reprocessing Technician RUMED (Level 1) certificate.
Participation Cost:
€ 80.00 per Module
Module Overview (Level I):
1.0 Standards, Laws and Regulation
2.0 Hygiene and Microbiology
3.0 Cleaning and Disinfection I
3.1 Cleaning and Disinfection II
4.0 Sterilization
5.0 Proper Machinery Working
6.0 Structure and Design of RUMED and its Processes
7.0 Quality Management and Validation I
8.0 RUMED Management I
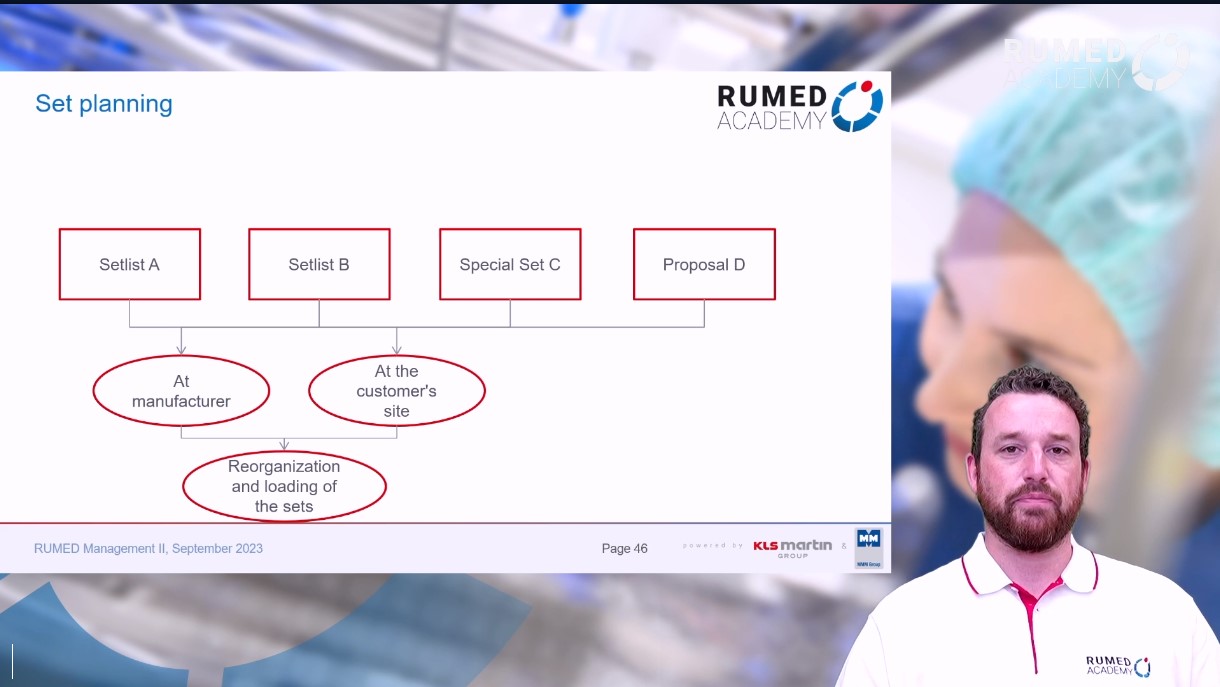
8.1 RUMED Management II
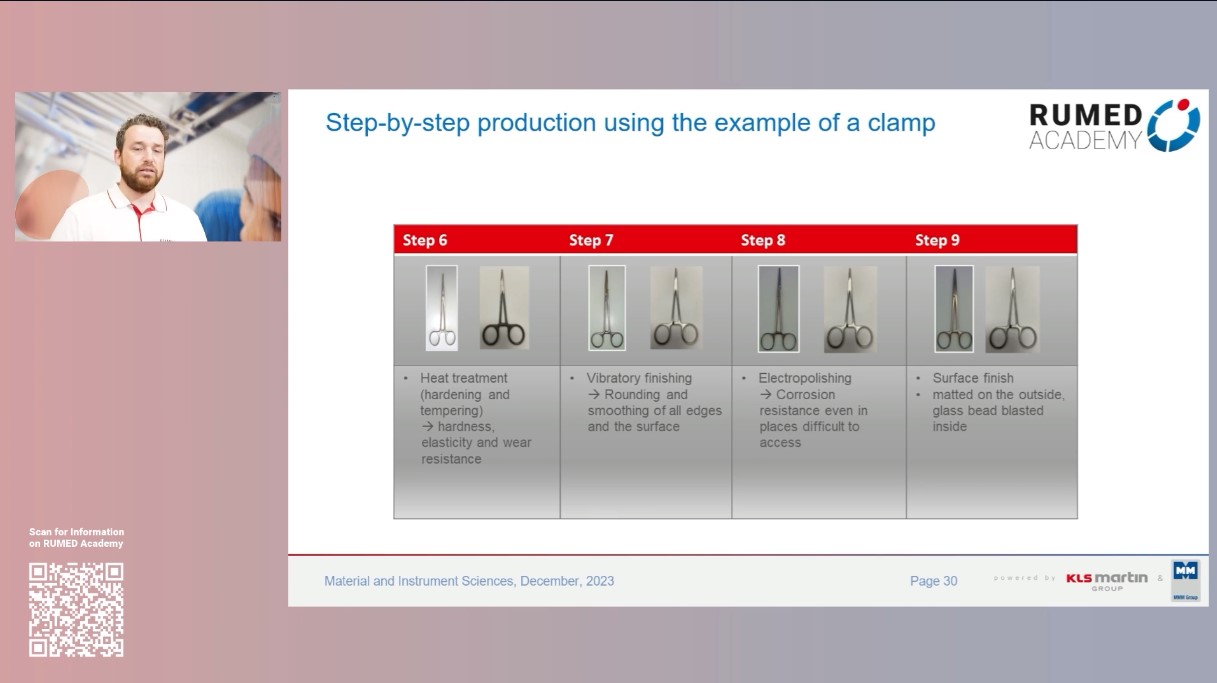
9.0 Material and Instrument Sciences I
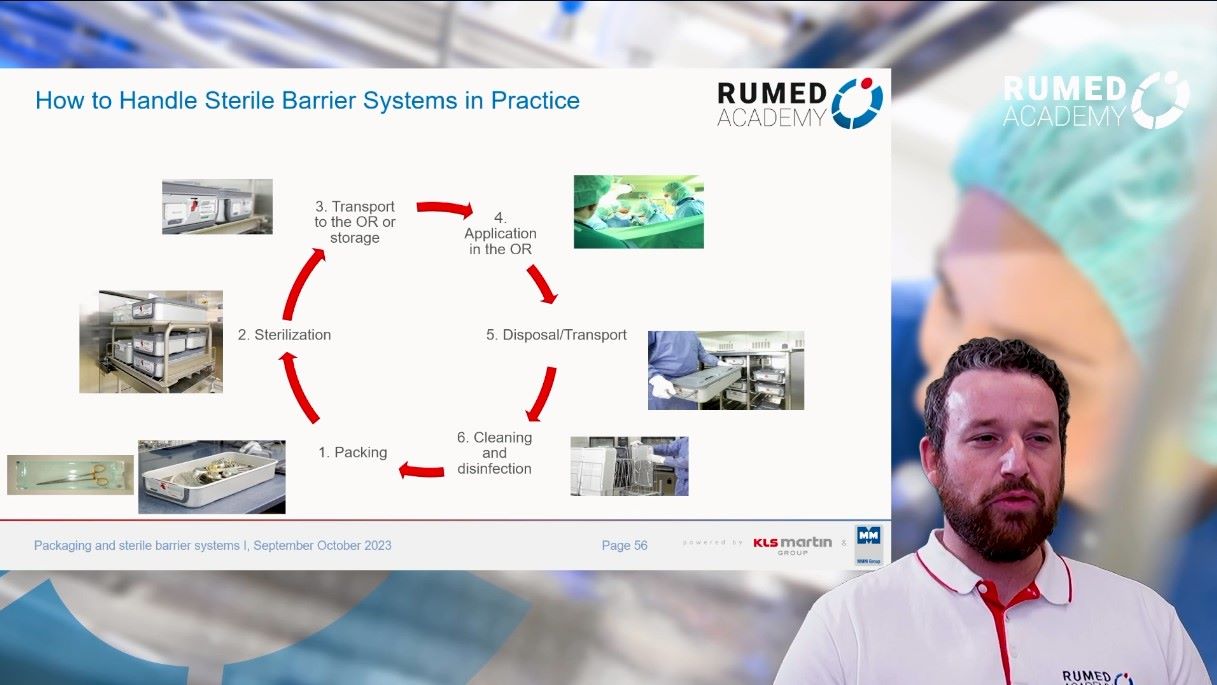
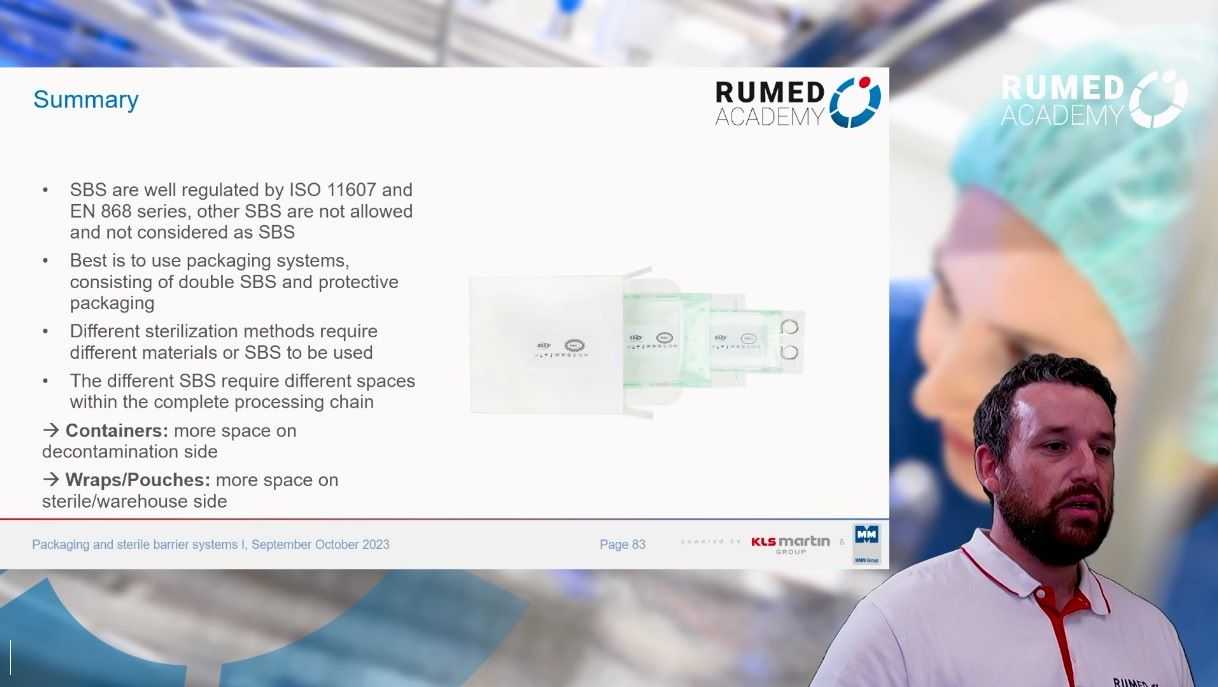
10.0 Packaging and Sterile Barrier Systems I
10.1 Packaging and Sterile Barrier Systems II
11.0 Processing of Medical Devices in the Supply Chain I