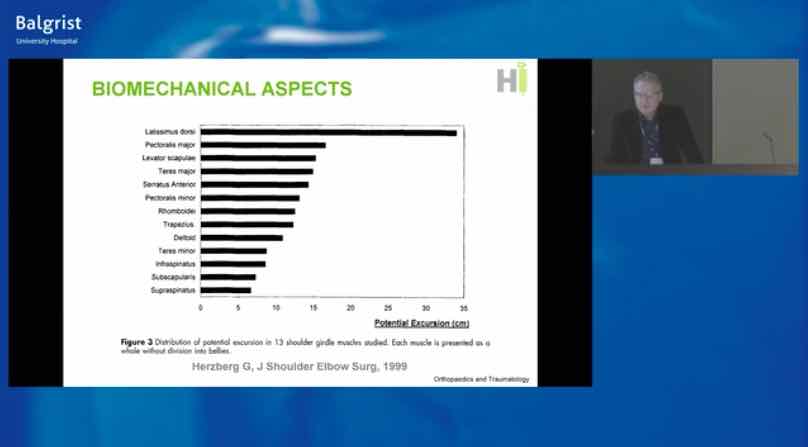
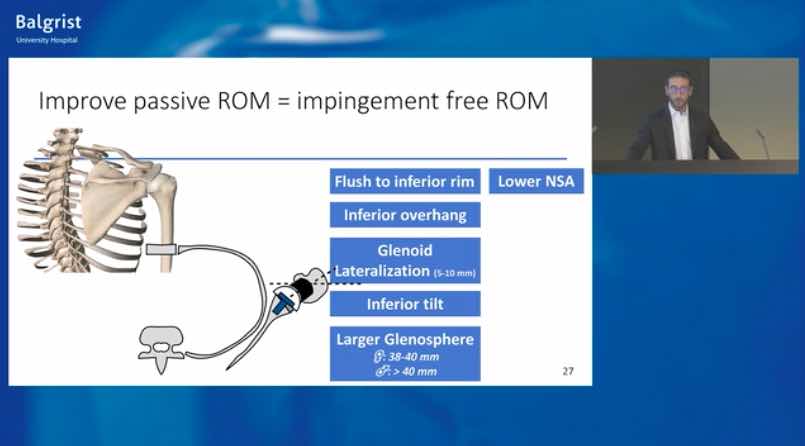
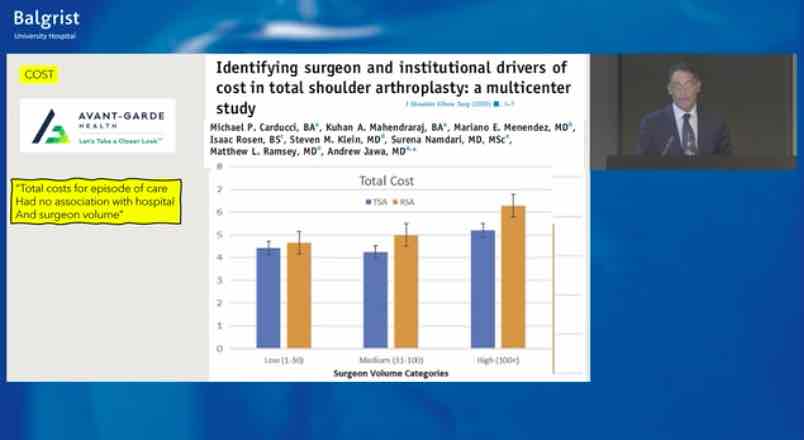
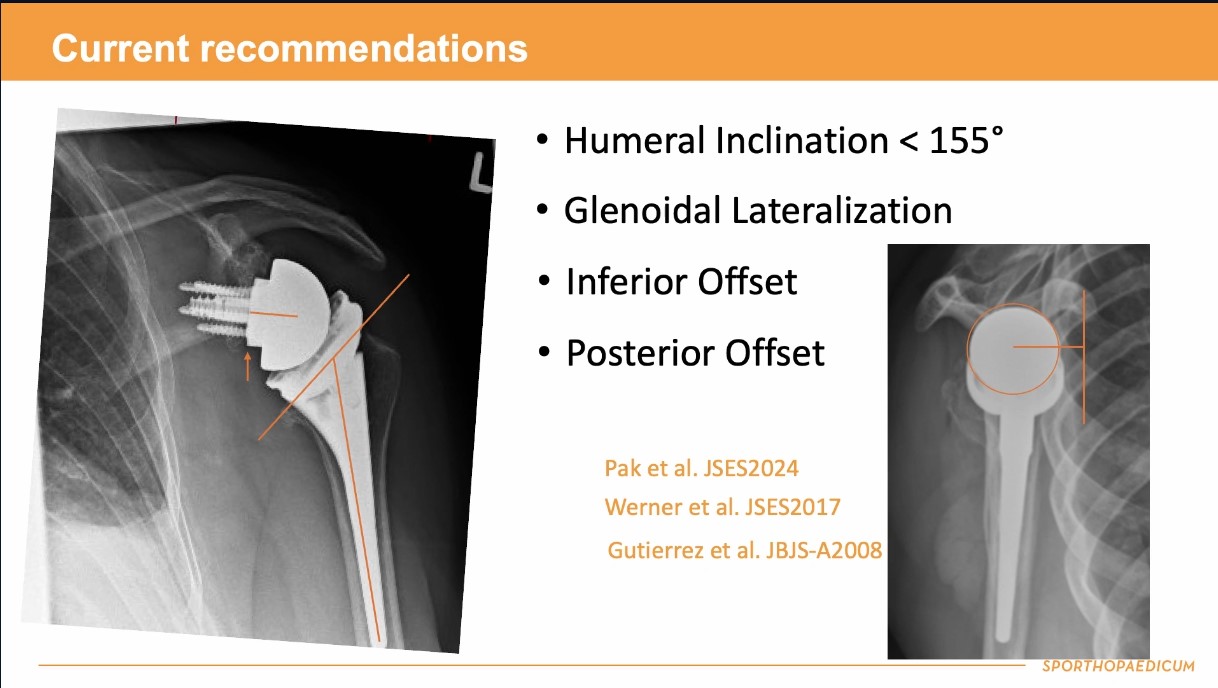
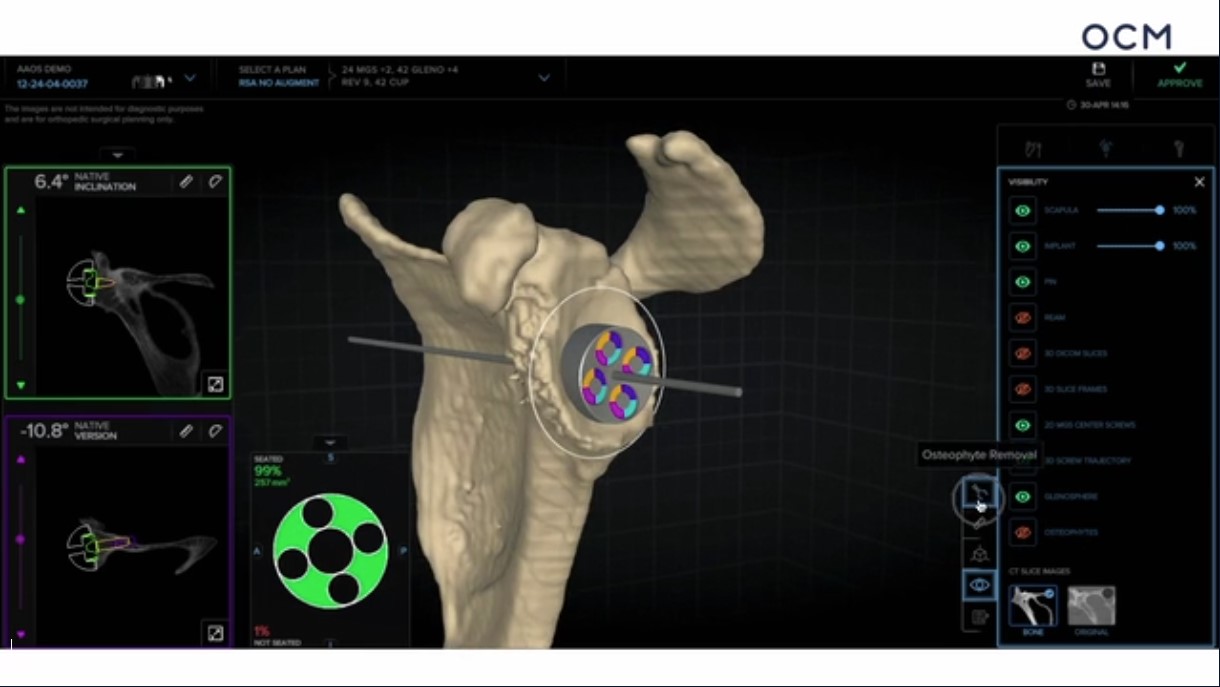
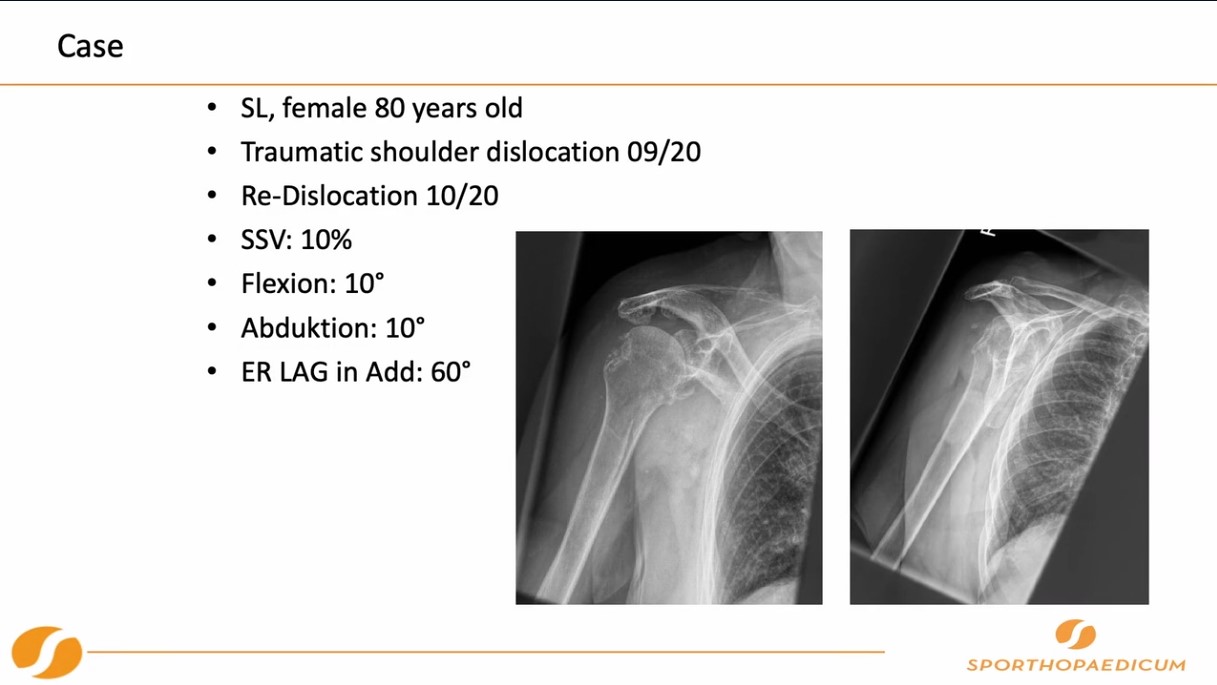
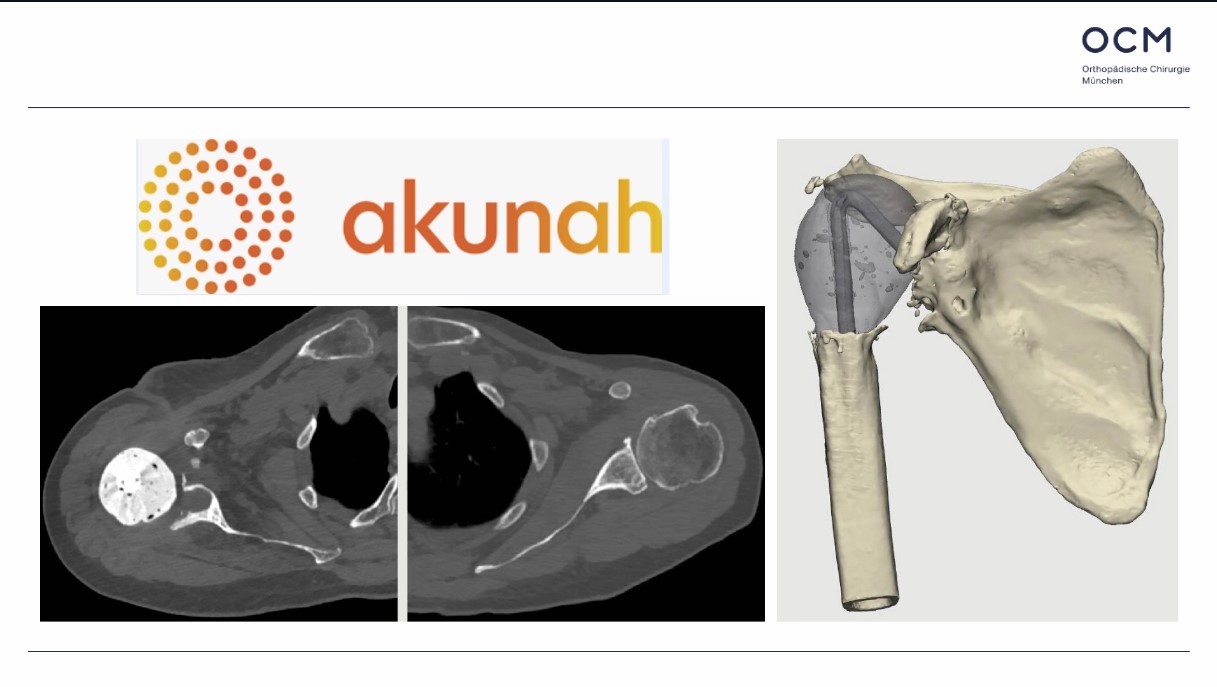
Already have a ticket? Log in here and open the event.
Home
Upcoming
Formats
- TV Events 95
- TechTips 18
- ScienceCast 34
- Expert Opinions 17
- DeepDives 6
- Broadcasts 60
Topics
Partners
Pricing
- Arthroscopy 153
- Cartilage 97
- Foot & Ankle 23
- Hand 2
- Hip 39
- Joint Replacement 82
- Knee 123
- Medical Device Reprocessing 46
- Neurology 0
- Non-Clinical 2
- Orthopedic Oncology 0
- Pediatric Orthopedics 0
- Phlebology & Lymphology 2
- Rehabilitation 13
- Shoulder & Elbow 56
- Spine 13
- Sports Medicine 156
- Trauma 18
Login
Signup
Home
Upcoming
Formats
- TV Events 95
- TechTips 18
- ScienceCast 34
- Expert Opinions 17
- DeepDives 6
- Broadcasts 60
Topics
Partners
Pricing
- Arthroscopy 153
- Cartilage 97
- Foot & Ankle 23
- Hand 2
- Hip 39
- Joint Replacement 82
- Knee 123
- Medical Device Reprocessing 46
- Neurology 0
- Non-Clinical 2
- Orthopedic Oncology 0
- Pediatric Orthopedics 0
- Phlebology & Lymphology 2
- Rehabilitation 13
- Shoulder & Elbow 56
- Spine 13
- Sports Medicine 156
- Trauma 18